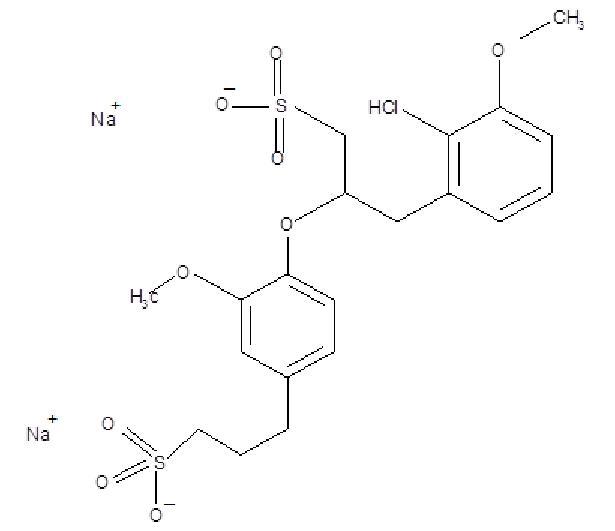
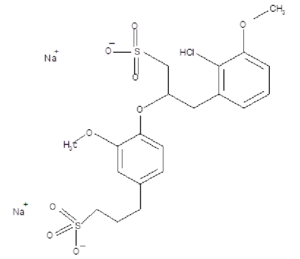
Sodium lignosulfonate Structure is a natural polymer has three aromatic alcohols coniferyl alcohols, p-coumaryl alcohol, sinapyl alcohol, coniferyl alcohols is the principla.
Three purified sodium lignosulfonates (SLs) with different molecular weights were prepared by ultrafiltration. The structural characteristics of SL and their effects on the properties of dye were investigated.
When the molecular weight is increased, the chroma, purity, and the content of guaiacyl of SL increases, whereas the phenolic hydroxyl, carboxyl, and sulfonic groups in SL decrease, the dispersibility, heat stability properties, and dyeing rate of SL on dye improve, and the fiber staining of SL weakens at high concentration. The fraction of SL with the cutoff molecular weight above 2.5 kDa has the weakest reduction effect on dyestuffs.
Molecular Weight:534.5 g/mol
MF: C20H24Na2O10S2
It has a strong dispersion capacity, due to the molecular weight and the type of functional group with varying degrees of dispersion capability.Also is a natural surfactant.
Sodium lignosulfonate has strong cation exchange capability, and also because of the presence of a variety of reactive groups on its organizational structure, and thus can produce condensation or hydrogen-bonding interactions occur with other compounds, also also -COOH, -OH function groups.
Sodium lignosulfonate is widely used in different area like chemical, construction, ceramics, the mineral powder metallurgy, pesticides, oil, carbon black, refractories, CWS dispersants other industries are widely promotion and application.
Most of the water-reducing agent is surface-active agent. Therefore, the mechanism of sodium lignosulfonate is the effect of surface activity.
Surfactants are organic compounds whose molecules have both hydrophilic and hydrophobic groups. Hydrophobic groups are various alkyl or alkaryl groups; hydrophilic groups are typically salts capable of dissociating ions. Depending on the dissociation of the ions by the hydrophilic groups, the surfactants can be classified into anionic surfactants; cationic surfactants; amphoteric surfactants; and nonionic surfactants.
When the surfactant is added into the aqueous solution, the surface tension (water-gas phase) and the interfacial tension (water-solid phase) of the water can be reduced, and the surface activity can be reduced. From the physical and chemical sense, the surface active agent refers to materials, when added to the aqueous solution, has its surface solution depth be greater than the depth of the solution itself, leaving the surface tension reduced.
After addition of the water reducing agent, lignosulfonate to the concrete, the water-repellent group of the water-reducing agent is directly adsorbed on the surface of the cement particles, and the hydrophilic group is directed to the aqueous solution, which makes up the adsorption film of single molecule or multi-molecule.
In the case, the cement particles are repelled and dispersed due to the same charge on the surface, releasing from excess water between particles to achieve the purpose of water reduction. At the same time, due to lowering the water surface tension and interfacial tension between the cement particles, while maintaining the same fluidity, the corresponding reduction in water consumption, and thus also play an effect on water reduction.