The spent liquor of the sulfite pulping process is dilute and contains other impurities; therefore, lignosulfonates should be separated from the spent liquor for increased commercial value. Because lignosulfonates are water-soluble products, they may not be precipitated by acidifying the spent liquor.
Membrane filtration has been recognized as a commercial process for recovering lignosulfonates from spent sulfite liquors because lignosulfonates have a higher molecular weight than that of other components in the spent liquors, and the difference in molecular weights allows for effective separation.
Ultrafiltration has been commercially applied in a calcium bisulfite pulping process[57] in Norway since 1981.[48,55] In this process, the spent liquor is filtered through a polysulfone ultrafiltration membrane with a surface area of 1120 m2 and a molecular weight cutoff of 20000 gmol@1. [48,55, 58] A report on this system stated concentration of the spent liquor from 12 wt% solids to 22 wt% solids at a flow rate of 50 m3h@1. [3,55] The retentate stream of this filtration contained up to 95% pure lignosulfonates, whereas the permeate mainly contained hemicelluloses.[47,48, 55] Lifespan is a crucial characteristic of membranes. With daily cleaning/maintenance, the lifespan of these membranes was claimed to be 12–15 months.[48,55] Cellulose acetate (at the temperature range of 50–60 8C) or Microdyn-Nadir UP010 membranes may also be used in this process.[3, 47]
However, there are drawbacks to using ultrafiltration to recover the lignosulfonates from the sulfite spent liquors. Despite worldwide use, ultrafiltration is not the most economical method for the separation of lignosulfonates, although it is the best of the current available commercial processes.[55] Pressure-driven membrane filtration operations are susceptible to membrane fouling and concentration polarization, which cause a decline in flux across the membrane and inhibit production capacity. [55] By implementing different-sized membranes to recover lignosulfonates, a more precise, efficient, and pure separation could be achieved. Additionally, the membrane separation method may not be selective to lignosulfonates due to a molecular weight overlap with hemicelluloses in solutions, which hinders the separation.
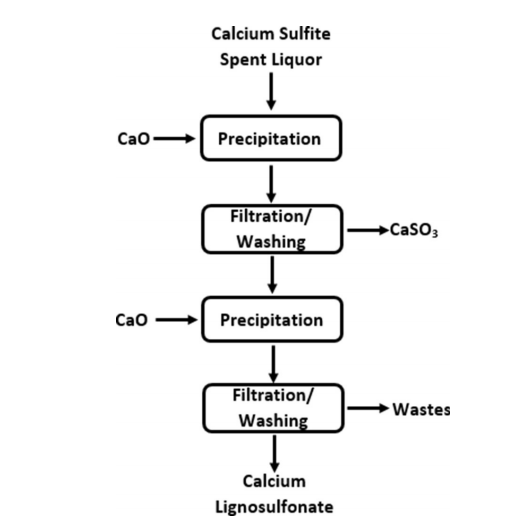
An alternative commercial method for recovering lignosulfonates is the Howard method.[56] Scheme 3 shows the Howard method, which may be employed in sulfite pulping processes by using calcium as the base. In this process, lime (calcium oxide) is added to the spent liquor initially to precipitate calcium sulfite at pH 8.5, which can be filtered and removed.[56,59] The filtered calcium sulfite may then be subjected to a pH change and further purified to regenerate pulping chemicals.[60] The addition of lime to the system in the following step, shown as the second precipitation stage in Scheme 3, leads to the production of calcium lignosulfonates, which are solid at a pH greater than 12.[61] As such, calcium lignosulfonates may be washed and filtered.[61] The recovery of lignosulfonate through the Howard method can be as high as 90– 95%.[62, 63]
Other methods for separating lignosulfonates from spent sulfite liquor have been outlined by Fatehi and Ni,[14] Fatehi and Chen,[3] and Lebo et al.[18] These include amine extraction, electrolysis, ion-exchange resin, the Pekilo process (fermentation and ultrafiltration), and reverse osmosis. In the amine extraction method, lignosulfonates are converted into water-insoluble lignosulfonic acid–amine adducts, which are then subjected to liquid–liquid extraction. [3, 14] Electrolysis involves the desalination and demineralization of magnesium sulfite spent liquor under conditions of 125 mAcm@2 at 60 8C for 3 h to produce lignosulfonates.[3, 64] Ion-exchange resin separation may use sandstone or limestone as an adsorbent for lignosulfonates.[3] Alternatively, ethanol can be used to precipitate lignosulfonates, which can be recovered through filtration and distillation.[3] Ethanol may also be recovered and reused in the precipitation stage.[3] The Pekilo process was introduced to ferment hemicelluloses of the spent liquor with Paecilomyces varioti. The treated spent liquor could then be subjected to ultrafiltration to produce >90% of pure lignosulfonates.[14] Reverse osmosis could also be employed to concentrate spent sulfite liquors.[65] However, these methods have not yet been commercialized, typically due to their high operating costs, which stem from solvent usage and purification of products.